European News
Varian, together with Maastro, a leading radiotherapy clinic in the Netherlands, recently launched an innovative brachytherapy applicator designed for accurate delivery of HDR brachytherapy in the treatment of rectal cancer, with the goal of reducing the quality-of-life risks associated with rectal surgery.*
This new partially 3D-printed applicator1 designed by Maastro radiation oncologists and physicists together with Varian experts to give radiotherapists access to improved treatment options.
“Removing rectal tumors surgically often has a huge impact on a patient’s quality of life. It may affect bowel function, sexual health, and some patients even have to live with a stoma,” says Dr. Maaike Berbée, radiation oncologist at Maastro.
Inspired by results from published studies on contact X-ray brachytherapy (CXB)2, which requires a dedicated, expensive machine for rectal cancer treatment, the Maastro team reached out to Varian early in the development process for help in co-developing a new design for a rectal cancer brachytherapy applicator suitable for contact brachytherapy. The goal was to deliver high-energy radiation directly to rectal tumors using a specially shaped applicator with multiple catheters for the highest possible precision, while taking advantage of Varian’s efficient and easy-to-use HDR brachytherapy treatment delivery system.
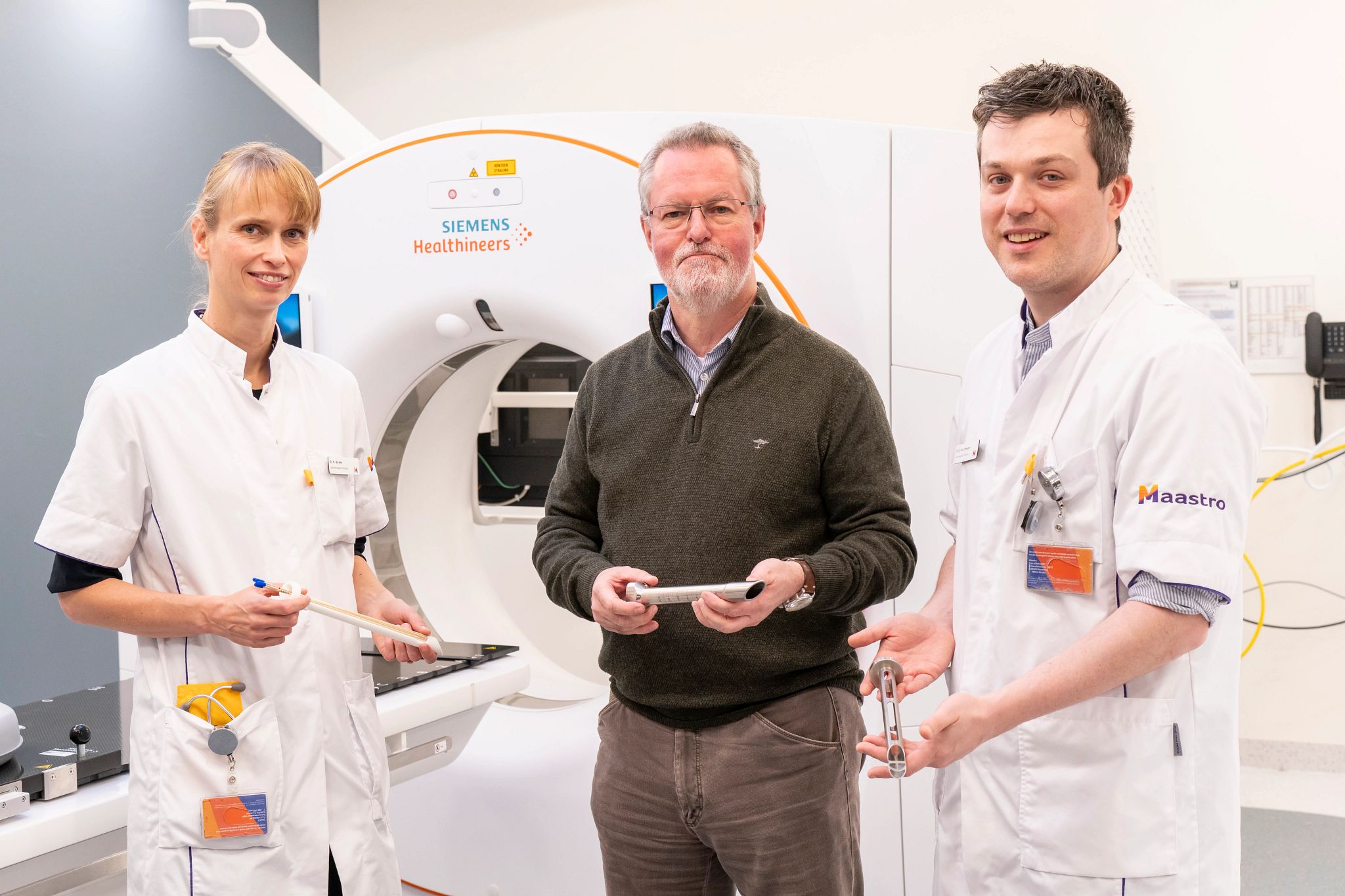
“An innovation such as this one is only going to be worthwhile if the technology can be used by radiation oncologists in as many clinics as possible,” said Prof. Frank Verhaegen, head of Maastro’s Physics Research Division. “We have been working with Varian equipment and applicators for years and developing this idea into a commercially feasible applicator that meets regulatory requirements and can be manufactured at scale, requires specific expertise.”
The applicator is designed to increase the contact surface with the tumor. It provides a uniform dose distribution to the tumor, with a dose profile that is similar to that of CXB, with a range from 60 Gy to more than 200 Gy EQD2, depending on tumor thickness. The included proctoscope assists with positioning and has integrated shielding to protect normal tissue.
“It opens up this type of treatment for many more patients,” said Marco Laumen, manager of Advanced Applicator Development at Varian. “This applicator works with our standard afterloader technology, which is used by clinics all over the world.”
The applicator has recently been CE (Conformité Européenne) marked and is ready for clinical use in countries that allow the use of CE marked technologies. As a next step, Maastro is planning a clinical trial to start later this year, designed to explore the clinical feasibility of the new HDR applicator for delivering results similar to CXB. The team is also looking at ways to achieve more clinical benefits.
“Following on from the trial, we’ll be looking into ways to tailor dose delivery to individual tumors and broadening the indication area and coming up with even more effective and individualized treatment strategies,” said Dr. Evert Van Limbergen, radiation oncologist at Maastro.
For more information, visit the Maastro website, or please contact your Varian brachytherapy sales rep.
**The new applicator, which has a CE marking and a Health Canada License, is available for use in Europe and other countries that require CE marking. It is not yet available in the United States or in countries that require FDA clearance, and its future availability cannot be guaranteed.
- Bellezzo M, Fonseca GP, Voncken R. et al. Advanced design, simulation, and dosimetry of a novel rectal applicator for contact brachytherapy with a conventional HDR 192Ir source. Brachytherapy. 2020 July-Aug;19(4):544-553. doi.org/10.1016/j.brachy.2020.03.009.
- Gerard JP, Barbet N, Schiappa R, et al. Neoadjuvant chemoradiotherapy with radiation dose escalation with contact x-ray brachytherapy boost or external beam radiotherapy boost for organ preservation in early cT2-cT3 rectal adenocarcinoma (OPERA): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2023 Apr;8(4):356-367. doi: 10.1016/S2468-1253(22)00392-2.