Early clinical cases using Varian’s IntelliBlate microwave ablation solution showcase the system’s intuitive set-up, ease of use and versatility, representing a powerful new tool for performing microwave ablation for interventional radiologists.
Ablation techniques – including microwave ablation – represent minimally invasive alternatives to surgery. Microwave ablation achieves its results by delivering focused energy to localized disease sites to destroy soft tissue and is an increasingly important part of a comprehensive portfolio of cancer care options.
A team at the University of California-San Diego was the first to use IntelliBlate in late August, performing a microwave ablation on a 67-year-old male with a metastatic lesion in the liver, who would have otherwise undergone surgery.
“I can’t tell you how proud I was that we were the first to perform an IntelliBlate case in the country,” shared Jonas Redmond, MD, Associate Clinical Professor of Radiology for University of California-San Diego, as he shared details about the case at the Western Angiographic & Interventional Society 2024 Annual Meeting. “There’s a lot to like about this system,” he added.
The IntelliBlate solution, which received 510(k) clearance from the U.S. Food and Drug Administration in July 2024, is the latest example of Varian's commitment to innovation in interventional solutions through continued investment in groundbreaking technology. Together with Siemens Healthineers, Varian is committed to connecting innovative therapy technologies and image-guided solutions. IntelliBlate serves as the cornerstone of a future-focused, integrated ecosystem for microwave ablation.
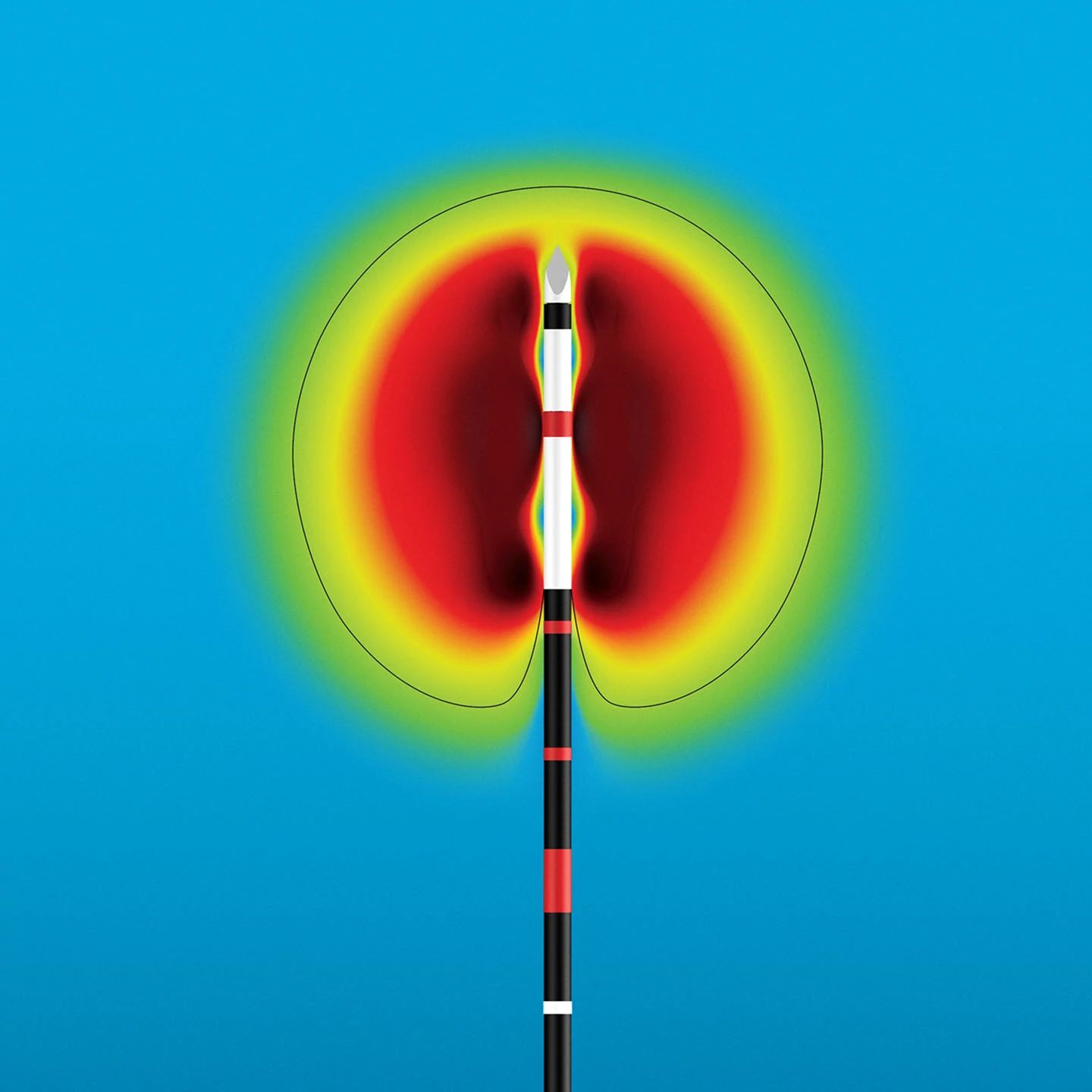
Dr. Redmond highlighted the sleek design and user interface of the IntelliBlate solution, making features easy to navigate and control. He also added “the fact that all ablation settings are right there on the system, with no need for external apps or workarounds, makes it incredibly easy to use. This system simplifies the entire ablation process—whether it’s adjusting the wattage or selecting the ablation width, it all happens with just a few touches, which allows us to focus on the patient, not fumbling with the technology."
Pointing to the simple set-up, which minimizes the risk of error, he added, “the ‘guide me’ function is fantastic for ensuring the setup is done right, especially for first-time users.”
Additionally, in terms of the ability to adjust both treatment time and watts, he stated “or just select the desired ablation width and have the system calculate everything, is really impressive,”
IntelliBlate produces large, controlled spherical ablation zones with the option to utilize a single or dual probes – each with linked or independent controls – “which is really useful in more complex cases,” Dr. Redmond shared, adding, “everything is customizable.”
The UCSD case was the first of more than two dozen cases across eight institutions as part of a limited market release of IntelliBlate and highlights the solution’s clinician-driven approach to interventional oncology, designed to streamline workflows and improve clinical outcomes. Clinicians in early cases noted that the IntelliBlate solution has a user interface that exceeds expectations, and that ease of set-up is better than their existing systems.
Jaimin Shah, MD, MBA, RPVI, System Associate Medical Director for Atlantic Medical Group in Morristown, New Jersey, used IntelliBlate for a handful of cases in September and October, including patients with cirrhosis who had undergone previous ablations. He highlighted the stiffness and steerability of the solution’s needle, adding “from setup to execution, IntelliBlate was straightforward and efficient—exactly what we need in a busy clinical setting.”
“The system’s ease of use, combined with its advanced features, makes it a standout in microwave ablation technology.”
̶ Abhishek Kumar, MD, Interventional Radiology Division Chief and Associate Professor at Rutgers University Hospital
Abhishek Kumar, MD, Interventional Radiology Division Chief and Associate Professor at Rutgers University Hospital, used IntelliBlate for a 73-year-old male patient with a mass in the left lobe and noted that he was able to easily select the desired ablation zone, including margins, directly on the interface and adjust once the needle was positioned in the patient.
“The system’s ease of use, combined with its advanced features, makes it a standout in microwave ablation technology.” Dr. Kumar said.
At NYU Langone, interventional radiologist Umair Lodhi, MD, used the system to treat a tumor in October, and rated system usability, needle tip sharpness and stiffness for ease of navigation and control during placement, exceeded expectations. Dr. Lodhi noted the enhanced visualization of the IntelliBlate solution’s needle under CT imaging once placed, adding “we saw a spherical ablation zone and got what we need to, without having to overtreat or damage healthy tissue."
Varian continues to evaluate early clinician experiences with IntelliBlate, with a full-market commercialization expected in early 2025, together with myAblation Guide by Siemens Healthineers.
Learn more about Varian’s IntelliBlate microwave ablation solution.